Leading frameworks for the pathology of AD
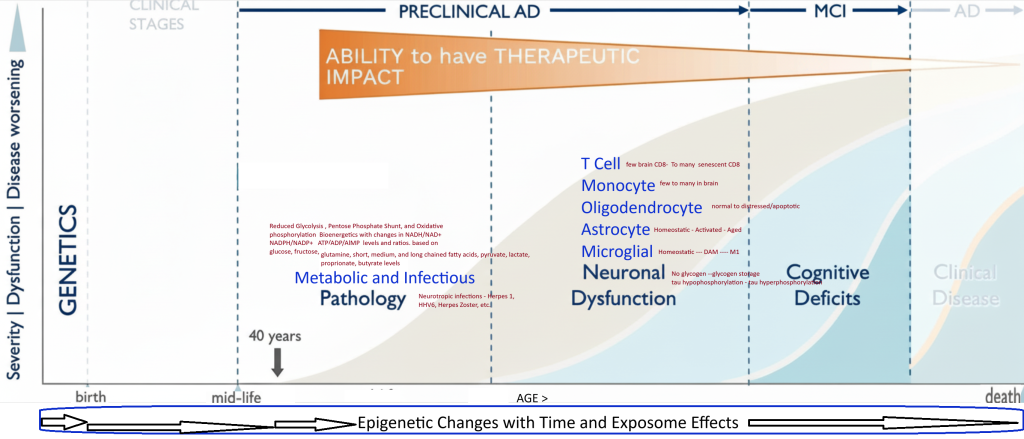
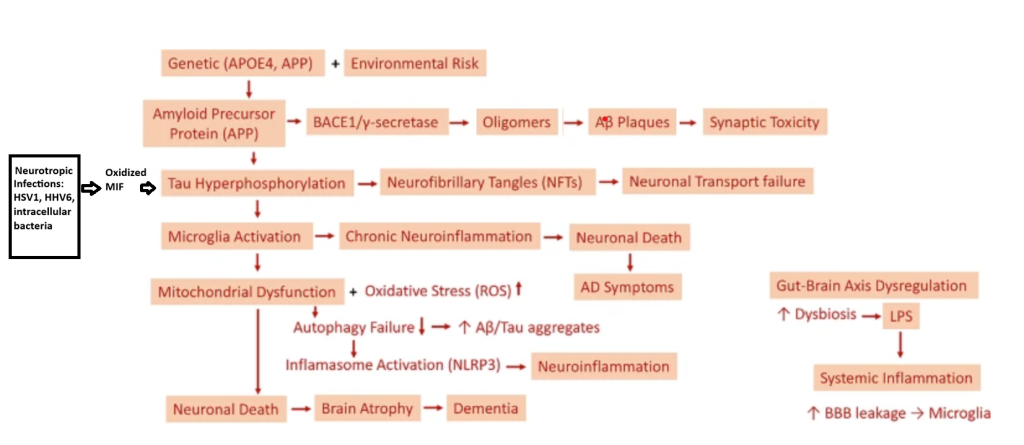
There are many genetically defined types of Alzheimer’s disease that can be subtyped into early or late onset. The following models primarily pertain to the late onset version of the disease and share the common endpoint pathology of Neurofibrillary Tangles, Lipid inclusions in Microglia, and Extracellular Amyloid Plaques.
- Complex Biological Systems based AD Model
- Biology of Aging based AD Model
- Multiple hit theory for the pathogenesis of AD
- Neurotropic Infection and Neuroimmunological Pathology
- Lithium , Iron, Copper, and Magnesium
- Metabolism and The Lipid Invasion
- Neuronal Excitotoxicity, Cholinergic Neuron Death, and Synaptic Connection
- Microbiota–Gut–Brain Axis providing Neurotoxins or Neuroprotective Agents
- Mitochondrial and Autophagy Dysfunction – See Biology of Aging Model
4. Specific Pathological Pathways to evaluate
- RNA splicing – circRNA and mRNA isoform switching
- Lysosomal System ( down regulated by AD )
- Exosome/Ectosome System ( down regulated by AD)
- Clathrin coated endosomal system – ( down regulated in AD)
- Immune System – Inflammation markers increased
- Mitochondrial – TCA , Electron Transport Chain, – use of fuel ( from glycogen-glucose to gluconeogenesis, or ketone use )
- Senescent State induction – astrocyte, neuron
- Antigen specific T and B cell creation and expansion
- Mitophagy
- Autophagy
- Phagocytosis
- Metabolism – glucose, lactate, butyrate, SCFA, LCFA, and pyruvate
- Proteostasis – ER stress, unfolding protein response, proteasomal activity, neddylation, ubiquitination
- Stress granule response
- Blood brain barrier
- Glymphatic flow
- Vascular disease
- Oxidation-Reduction- ROS
- Neural Networks
- Synaptogenesis
- Nano plastics in the brain